
ISO 13485 standard is an effective solution to meet the comprehensive requirements for Medical Devices Quality Management System. Adopting ISO 13485 provides a practical foundation for manufacturers to address the Medical Device Directives, regulations and obligations as well as demonstrating a commitment to the safety and quality of medical devices.
What is ISO 13485?
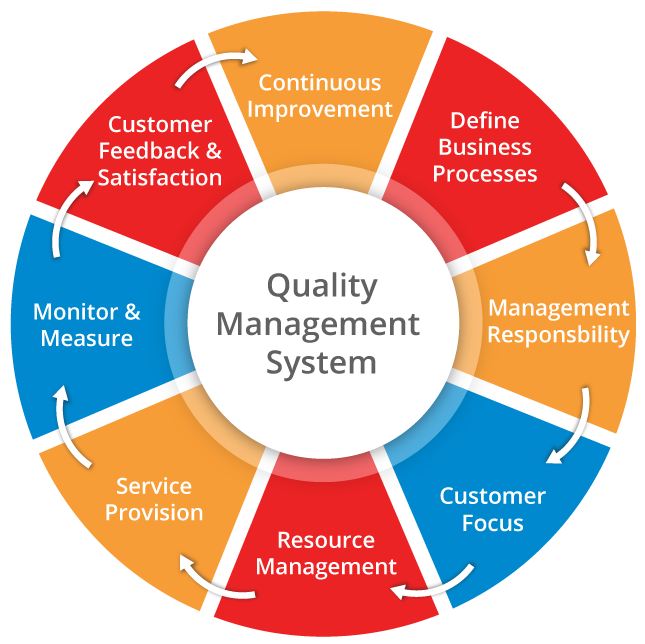
ISO 13485 was established to support medical device manufacturers in designing quality management system that identifies and maintains the effectiveness of their processes. It designed to be used throughout the life cycle of the medical device; from initial inception to production and postproduction, including final decommission and disposal. It also covers aspects such as storage, distribution, installation, servicing and the provision of associated services.
ISO 13485 is a stand-alone Quality Management System standard, derived from the internationally recognised and accepted ISO 9000 quality management standard series. ISO 13485 adapts the ISO 9000 process-based model for a regulated medical device manufacturing environment including their critical suppliers and distributors. While ISO 13485 is based on the ISO 9001 process model concepts of Plan, Do, Check, Act, it is designed for regulatory compliance including the requirements set by the MHRA and MDR. It is more prescriptive in nature and requires a more thoroughly documented quality management system.

The benefits of ISO 13485?
ISO 13485 specifies requirements for a quality management system where an organisation needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements. ISO 13485 can also be used where organisations are involved in one or more stages of the life-cycle, including design and development, production, storage and distribution, installation, or servicing of a medical device or provision of associated activities (e.g. technical support). It can also be used by critical suppliers or external parties that provide product, including quality management system-related services to such organisations.
ISO 13485 can help you improve overall performance, optimise efficiency, eliminate uncertainty, and widen market opportunities. Companies with this certification communicate a commitment to quality to both customers and regulators.
Other main benefits include:
- It provides the manufacturer with a higher level of confidence in the ability to consistently achieve and maintain compliance with regulatory requirements.
- It can also help to minimize risks and failures which might adversely affect patient safety and damage a manufacturer's reputation.
- ISO 13485 is the best globally accepted Quality Management System a medical device organisation can implement to help demonstrate compliance with laws and regulations of the medical device industry.
- It is the quality management system standard accepted as the basis for CE marking medical devices under European Directives and Regulations.
Why choose SMART QMS for your ISO 13485 implementation?
At Smart QMS, we are committed to providing our clients with the best possible services. we have experienced consultants specialised in Medical Devices industries. Together with exceptional ISO consultants, we work closely with our clients and a rigorously selected group of technical experts to ensure you receive the best possible service in your project as well as the most adequate guidance and advice. We will develop compliant policies and procedures for your organisation.
We are committed to providing our clients with the best possible value for money – including a price promise from the outset.
Our goal as an organisation is to have a customer service that is not just the best but legendary.

